eSubmissions lets users of Activator send messages to the FDA via the AS2 message protocol. Using eSubmissions requires a proper license.xml file and FDA approval of trading partners.
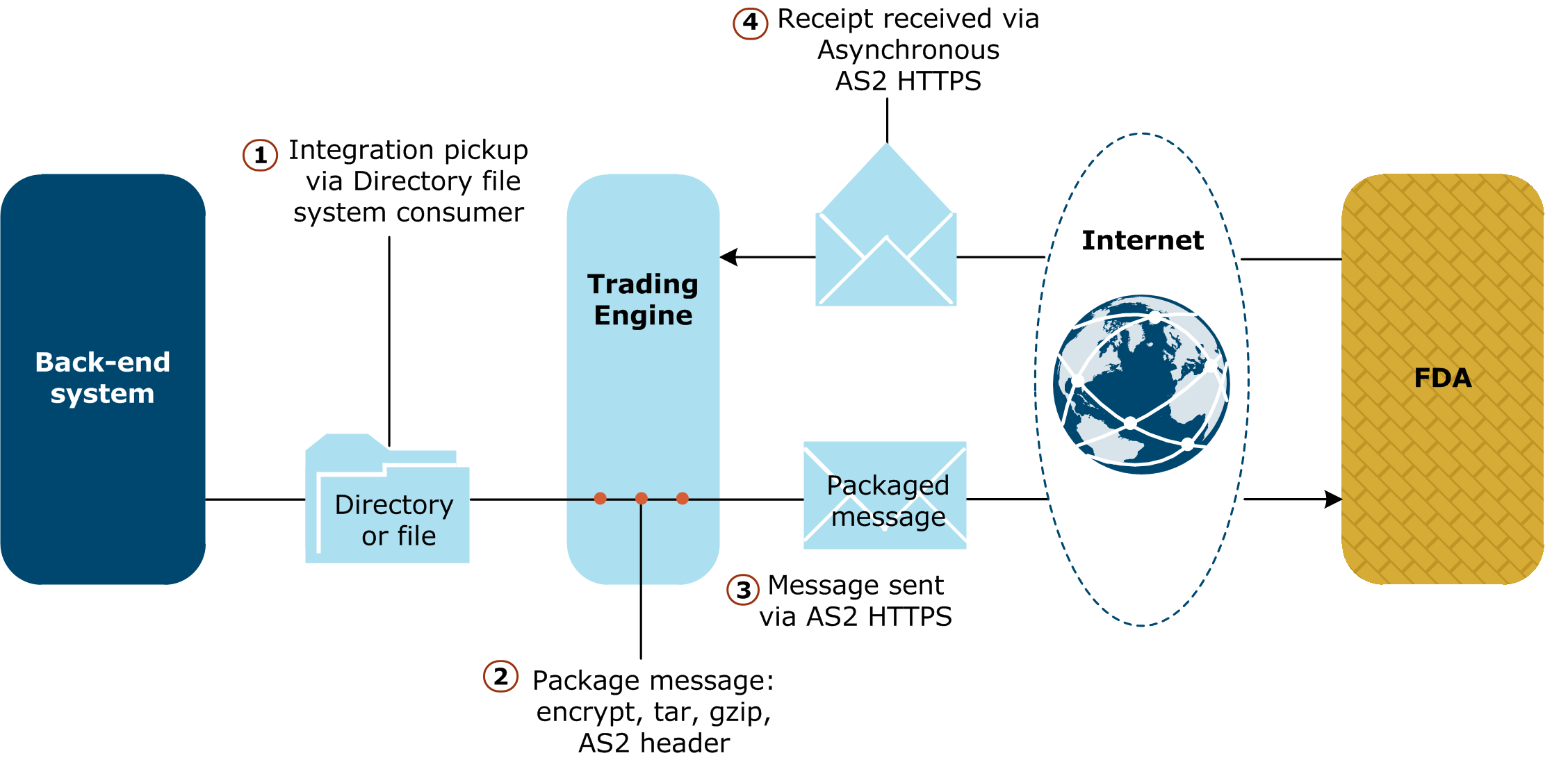
Intended primarily for sending large messages of up to 8 gigabytes or more, eSubmissions has payload versatility. You can send a single small file or a directory containing many subdirectories and files. Regardless of size, Activator securely packages and compresses all outbound messages the same way. The following illustrates the process for sending messages to the FDA using eSubmissions.

A key component of eSubmissions is a custom application pickup named Directory file system consumer. This transport handles only messages outbound to the FDA. In addition, eSubmissions adds two metadata elements — FdaCenter and FdaSubmissionType — to the AS2 MIME headers of outbound messages. Values of these elements identify the messages’ intended destinations and categories.
You can monitor eSubmissions message activity in Message Tracker, just as you can follow the processing trail for any type of e-commerce traffic.